 There are small amounts of sugar in all whiskies. Scottish whiskies have some sugars dissolved from the oak cask and often some from optional caramel colouring (E150a). Total amount of sugars is quite low, usually well below 1 g/l, but in certain cases it is quite possible to reach a few grams per liter. The sweet aromas of whisky matured in refill bourbon or new oak casks mostly come from sweet aromatic vanillin and fruity esters, not from the sugars. However, sugars can have a significant role in the case of casks previously used for sweet wine or sweetened spirit.
There are small amounts of sugar in all whiskies. Scottish whiskies have some sugars dissolved from the oak cask and often some from optional caramel colouring (E150a). Total amount of sugars is quite low, usually well below 1 g/l, but in certain cases it is quite possible to reach a few grams per liter. The sweet aromas of whisky matured in refill bourbon or new oak casks mostly come from sweet aromatic vanillin and fruity esters, not from the sugars. However, sugars can have a significant role in the case of casks previously used for sweet wine or sweetened spirit. |
| Typical composition of a liquid E150a colouring |
 |
| Caramel content of various whiskies (Boscolo et al 2002) |
Another source of sugars in whisky is wood. Oak is composed of three main macromolecules; cellulose is a long-chain D-glucose-fiber. Hemicellulose is a mesh-like branched chain of 5-carbon sugars (xylose, arabinose, galactose, ribose, rhamnose) and glucose. Lignin is a complex matrix of polypropane and polyphenols. Cellulose breaks down very slowly in alcohol-water solution extracting very small amounts of glucose to whisky. Most sugars extracted from the cask come from hemicellulose, hence most of them are 5-carbon sugars. Arabinose, xylose and rhamnose taste about half as sweet as glucose, where as fructose is almost twice as sweet compared to glucose.
About 100 mg/l of solids (including sugars) is extracted from new charred oak casks during the first year of bourbon maturation and after 1-3 years typically significantly less and only as little as 4 mg/l/year extraction has been reported from old refill casks used for example for cognac (or Scotch) maturation. A toasted cask extracts considerably more sugars compared to a charred cask, but typically about 150-250 mg/l/year is reached for the first year(s) of maturation. For comparison, a 40 years old brandy matured in a toasted oak cask had 2 g/l sugars (averaging 50 mg/l/year), while a 30 years old cognac (probably mostly matured in a refill casks) had only 0.5 g/l (17 mg/l/year).
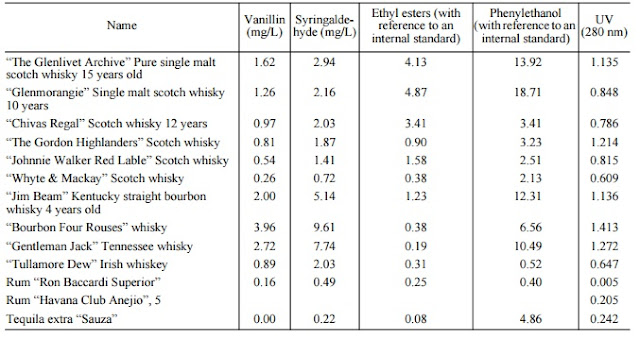 |
| Analysis of different spirits by GC-MS (Savchuk 2001) Note the high levels of vanillin from new charred casks. |
 |
| Venezuelan rum, 40 g/l sugars |
 |
| Pedro Ximenez sherry, 470 g/l sugars |
The Scotch whisky industry has preferred sweet sherries for seasoning of their whisky casks from the 19th century and while most modern sherries are quite dry, most ex-sherry whisky casks were used for (or seasoned with) sweet sherries. In the 1800s and the early 1900s sweet(ened) sherries were imported to Britain in shipping casks. Britain was a major importer of sherry and emptied shipping casks were often used for maturation of Scotch whisky. By the late 19th century sherry casks were used extensively in Scotland and the first attempts to rejuvenate or season used casks were made in the 1890s. After the sherry casks were used for Scotch whisky maturation, they were seasoned with sweet sherry or paxarette, sometimes using pressure to soak the wood with the wine. By the early 20th century the use of sherry casks was quite wide spread, for example in the late 1920s a big whisky blender Johnnie Walker matured all of their whiskies in sherry casks or sherry-treated casks.
 |
| Aberlour 18yo sugars <2 g/l |
Shipping casks were mostly Spanish coopered lightly toasted American oak (Quercus alba) butts or puncheons (often called bocoyes in Spain). In 1972 Manuel González Gordon stated that "in recent years some Spanish oak has been used, due principally to the difficulties of importing American timber, but its greater density and hardness and lower porosity make it less suitable than the American wood". In the 19th century and the early 20th century they were often used for fermentation prior to shipping. Oak influence was not that detrimental for sherry during fermentation period and the fermentation process was believed to extract some of the excess tannins and bitterness from the wood prior the use in a solera or shipping.
The fermentation phase added plenty of sugars to the oak. For example, a typical 1980s (arguably Spanish oak, but at least Spanish coopered oak) shipping cask previously used for fermentation extracted 2.25 g/l sugars to whisky during only 6 years of maturation (375 mg/l/year). On the other hand an American ammonia-treated cask without a previous fermentation use would extract 770 mg/l (154 mg/l/year) sugars from a fino-seasoned cask and even less 655 mg/l (133g/l/year) from a similar oloroso cask. According to the table below, the previous use as a fermentation cask is very significant in the terms of sugar extraction.
 |
| JM Philp 1989 |
 |
| Ardbeg Dark Cove 4 g/l sugars |
REFERENCES AND FURTHER READING:
Alañón ME et al. Monosaccharide anhydrides, new markers of toasted oak wood used for ageing wines and distillates. Food Chem 2010;119;505-512
Blanco Gomis D et al. Evolution of sugars in cider brandy aged in oak barrels, a contribution to its characterization. J Agric Food Chem 2003;51;923-926
Boscolo M et al. Spectrophotometric determination of caramel content in spirits aged in oak casks. J AOAC Int 2002;85(3);744-750
Boudet AM et al. Biochemistry and molecular biology of lignification. New Phytol 199;129;203-236
Clyne J et al. The effect of cask charring on Scotch whisky maturation. Int J Food Sci Tech 1993;28;69-81
González Gordon M, Sherry. Cassell Ltd 1972
Haldane FF. Casks; their manufacture and treatment. J Inst Brew 1906;12(7);688-711
Hills, P. Appreciating whisky. Collins 2000
Kansallisarkisto, Helsinki. http://www.arkisto.fi/en/the-national-archives-service/arkistolaitoksen-vaiheet-2
Liebmann AJ & Rosenblatt M. Changes in whisky while maturing. Ind Eng Chem 1943;35(9);994-1002
Martínez Montero C. Estudio de parámetros alternativos como indicatores del envejecimiento y de la calidad del brandy de Jerez. Thesis, Universidad de Cádiz, 2006
Martínez Montero C et al. Sugar contents of brandy de Jerez during aging. J Agric Food Chem 2005;53;1058-1064
Mosedale, JR. Effects of oak wood on the maturation of alcoholic beverages with particular reference to whisky. Forestry 1995; 68; 3; 203-230
Mosedale JR & Puech JL. Wood maturation of distilled beverages. Trends Food Sci Tech 1998;9;95-101
Mosedale JR & Puech JL. Wood maturation of distilled beverages. Trends Food Sci Tech 1998;9;95-101
Piggott JR et al. Effects on scotch whisky composition and flavour of maturation in oak casks with varying histories. Int J Food Sci Tech 1993;28;303-318
Piggott, JR et al(ed). The Science and technology of whiskies. Longman 1989
Piggott, JR et al(ed). The Science and technology of whiskies. Longman 1989
Read J. Sherry and the sherry bodegas. Sotheby's 1988
Savchuk SA et al. Application of Chromatography and Spectometry to the Authentication of Alcoholic Beverages. J Anal Chem 2001;56(3);246-264
Tolman LM & Trescot TC. A study of the methods for the determination of esters, aldehydes and furfural in whisky. J Am Chem Soc 1906;28(11);1619-1630
Tsai PJ et al. Interactive role of color and antioxidant capacity in caramels. Food Res Int 2009;42;380-386
Valaer P & Frazier WH. Changes in whisky stored for four years. Ind Eng Chem 1936;Jan;92-105
Valaer P. Scotch whisky. Ind Eng Chem 1940;32(7);935-943
Valaer P. Foreign and domestic Rum. Ind Eng Chem 1937;Sep;988-1001
Williams JF. Rapid determination of alcohol in distilled spirits and of color in whisky. Ind Eng Chem 1926;Aug;841-843
Savchuk SA et al. Application of Chromatography and Spectometry to the Authentication of Alcoholic Beverages. J Anal Chem 2001;56(3);246-264
Tolman LM & Trescot TC. A study of the methods for the determination of esters, aldehydes and furfural in whisky. J Am Chem Soc 1906;28(11);1619-1630
Tsai PJ et al. Interactive role of color and antioxidant capacity in caramels. Food Res Int 2009;42;380-386
Valaer P & Frazier WH. Changes in whisky stored for four years. Ind Eng Chem 1936;Jan;92-105
Valaer P. Scotch whisky. Ind Eng Chem 1940;32(7);935-943
Valaer P. Foreign and domestic Rum. Ind Eng Chem 1937;Sep;988-1001
Williams JF. Rapid determination of alcohol in distilled spirits and of color in whisky. Ind Eng Chem 1926;Aug;841-843































