|
| Same whisky, chilled with a drop of water on the left |
The main reasons for filtering the spirits are removing any solid components, improving the flavour and avoiding the haze formation later in the bottle or glass.
Spirits have been chill filtered at least from the late 14th century. Russian vodka was traditionally rapidly cooled after distillation with ice. The excess water transformed into ice and most of the oils hardened on top of that. Filtering became common before the 17th century. The early filters were usually made of cloth (felt, cotton), but also of paper, sand and charcoal. Chemical purification was also used quite early on, just as with wine, mead or beer. Spirit cleansing additives of the 17th and 18th century included ash, potash, burnt wormwood, and the gentry also used milk, eggs, fresh black bread, soda and isinglass to produce top quality vodka.
 |
| Charcoal in Jack Daniel's distillery (alcademics.com) |
 |
| Sand and coal filter tanks in Zhitomir vodka distillery |
| EU limit [g/l] | Modern spirit, est. | Analysis 1905 | Analysis 1966 | |
| Scotch malt | 10 | ~ 1 | 1,5-3,5 | 2,7-3,9 |
| Scotch blend | 10 | <1 | 0,5-1,9 | 1,7-2,2 |
| Scotch grain | 10 | <1 | 0,5-3,0 | 0,7-1,3 |
| Bourbon | 10 | ~ 1,5 | 5,9-6,8 | |
| Rice spirits | 10 | 2-5 | ||
| Rum | 2,25 | |||
| Fruit brandies | 2 | |||
| Marc | 1,4 | 3,5-4,5 | ||
| Brandy | 1,25 | <1 | 1,5-5,7 | |
| Canadian whisky | 10 | 1,15 | ||
| Irish whisky | 10 | 6,95 |
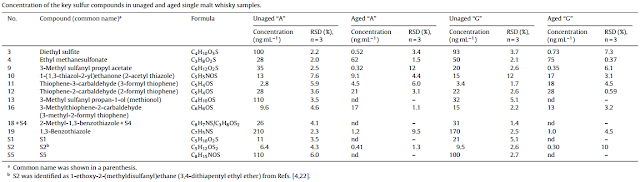
The haze formation occurs when the long chain fatty esters become insoluble in lower temperatures or at lower alcoholic strengths. The most crucial esters in haze formation in whiskies are ethyl laureate (ethyl-dodecanoate), ethyl-palmitate (ethyl-hexadecanoate) and ethyl-9-hexadeanoate (ethyl-palmitoleate). Alcocol content of 45% abv is believed to be a crucial limit for these long chain esters to percipitate in room temperature. Other compounds responsible for the cloudiness are high molecular weight lipids, especially sitosterol beta-D-glucoside (probably cask-derived) and slightly ethanol-soluble lignins from the cask.
The haze-forming long chain esters are not very aromatic, ethyl laureate gives some floral, fruity and waxy aromas, as ethyl palmitate and ethyl palmitoleate mostly contribute to the mouthfeel (waxy, oily), although they are reported to give some aromas of coconut and fruits. Probably the more important factor is their ability to act as a surfactant and to enhance/suppress other aromas.
(Coal) filtering increases the formation of short esters and the conversion of aldehydes and ketones to alcohols, probably because of the slight oxiditation caused by the process. On the other hand, some (7%) ethyl acetate (pear-drops) was removed in a typical Scotch filtration, so the net effect remains unclear. The highly efficient nanofiltration and active coal filtration techniques used by brandy and vodka industries remove great deal of long esters and some terpenes. Even a light filtration removed all of the nerol (rose,lemongrass) from apricot brandy and the same probably happens in Scotch whisky filtration. Studies on Glenlivet malt whisky indicate that significant amount of gallic acid was removed in filtering, but the same study found no difference on nosing aroma.
 |
| Plate and frame filter in a bottling plant |
 |
| Plate and frame filter (whisky.com) |
References and further reading.
Braus H et al. Isolation and identification of a sterol glucoside from whiskey. Agr Food Chem 1957;5(6);458-9
Da Porto C. Effects of chill filtration on the composition of grape spirit. Wein-Wissenschaft 2000;55(1);7-12
Duarte FC et al. Physicochemical and sensory changes in aged sugarcane spirit submitted to filtering with activated carbon filter. Ciênc Tecnol Aliment.,2012;32(3);471-477
Glaub, R et al. Effects of various filter systems on sensory quality of fruit brandies. Kleinbrennerei 1998;50 (1);6–12
Himmelstein L. The king of vodka. Harper 2010
Hsieh CW et al. Develop a novel method for removing fusel alcohols from rice spirits using nanofiltration. Food Sci 2010;75(2); 25-29
Ko W et al. Removal of higher fatty acid esters from Taiwanese rice-spirits by nanofiltration. p353. In Distilled spirits, ed Walker GM et al NOttingham Univ Press 2012.
Lachenmeier D et al. Defining maximum levels of higher alcohols in alcoholic beverages and surrogate alcohol products. Reg Tox Pharm 2008;50;313-321
Miljic UD et al. The application of sheet filters in treatment of fruit brandy after cold stabilisation. Acta per tech 2002;44;87-93
Taylor AJ, Mottam DS. Flavour science: Recent developments, RCS 1996
Persson KM. Med kol och kolonn. Spiritus 2005;7
Piggott JR et ak. The science and technology of whiskies. Longman 1989
Pirie G et al. Membrane filtration of whisky. Food & Drink 2000; p9-13. IChemE 2000.
Pokhlebkin W. A history of vodka. Verso, 1992
Puskas V et al. Influence of cold stabilisation and chill membrane filtration on volatile compounds of apricot brandy. Food Bioprod Proc 2013;91;348-351
Schidrowitz P, Kaye F. The determination of higher alcohols in spirits. Analyst 1906;31;181-194
Singer DD. The analysis and composition of potable spirits. Analyst 1966;91;127-134
Wisniewski I. Filtering out. Whisky Magazine 2011;97;27
Malt Maniacs E-pistle: http://www.maltmaniacs.net/E-pistles/Malt-Maniacs-2011-06-Chill-filtration-and-cloud-formation-in-whisky.pdf
Whisky.com study: http://www.whisky.com/information/knowledge/science/study-on-the-chill-filtration-of-scotch-single-malt-whiskies.html