|
| Glenfiddich still room |
Copper is the traditional material for Scottish stills, but the first distillers of aqua vitae were most likely using pots of clay or glass. In Asia, clay and porcelain pots with bamboo condensers were used. Quite minute quantities of spirits were prepared before the 14th century. As metallurgy improved and distilling became a larger scale operation, the stills were forged from tin, iron, brass and copper. Copper was easiest to keep clean, not too heavy and relatively easy to forge, so it became the common still material, at least in Britain and France quite early on, probably in the 15th century.
 |
| Indian still from 18th century |
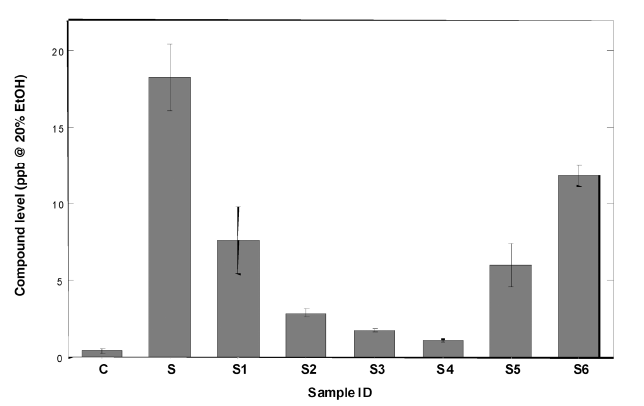
Copper is often claimed to suppress the amount of sulphury compounds in the spirit, but there is really quite a little research or even theoretical understanding about the phenomenon. The most predominant effect promoting the sulphury aroma in spirits is dimethyl trisuphide (DMTS). Its perception treshold is about 0,1µg/litre and the typical concentrations in spirits vary between 1-6µg/litre. The amount of DMTS has been shown to diminish in the spirit by the copper influence. However, not just any copper influence has that effect. For example, by using copper salts in a glass still increases DMTS, but copper wool in a glass still decreases DMTS, just not as much as a copper still. In addition, an used and patinated copper still seems to be more effective in DMTS-reduction compared to clean copper. To make things even more complex, the other metal ions and the antioxidant potential of the wash change the settings of the system once more. For example, iron decreases the copper effect, but ascorbate (by acid or antioxidant effect?) increases the DMTS formation. Most common aromatic sulphury compounds present in the wash are (pot) distilled just about in the same quantities over to the spirit, whether a copper or steel still is used, including DMS, DMDS, MMFDS, thiophene, thianaphthene and S-methyl thioacetate.
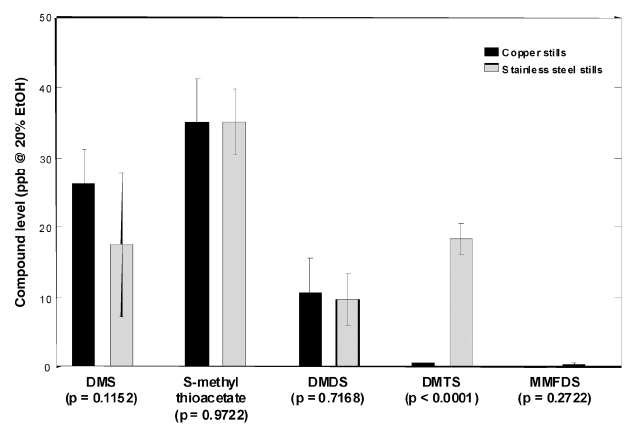 |
| Levels of sulphur compounds in new make from copper and stainless steel stills (Harrison 2011) |
There is some evidence of increased ester formation from acids and alcohols during the distillation, but that may happen in higher temperatures anyway and have nothing to do with the copper. Copper seems to have some effect on phenols, decreasing slightly the amount in the distilled spirit.
 |
| Copper plates in a column still |
The most influential part of distillation in terms of sulphur (DMTS) removal is in the spirit still body and interestingly in the vapour phase of the wash still, but not as much in the wash still body or the spirit still vapour phase. Alcohol concentration might play a role in the catalyst properties of copper. The spirit still condensers at the end of the second distillation have the least effect to the DMTS removal, on the other hand (at least in the column stills) the late phase condensers are shown to be important in conversion of dimethyl sulphide (DMS) to less aromatic sulphide.
Reflux is important factor in copper influence. It means the amount of vapour condensed and trickled back down to the still from the head and lyne arm instead over to the condenser. Reflux depends on the charge (fill level) of the still, whether the wash is cooled or preheated, the shape of the still, the speed of distillation and the cooling of the still and the condensers. High reflux is achieved by low preheated charge, big tall narrow stills with boiling ball and steam coils, ascending lyne arm and efficiently cooled tube condensers. High reflux means lower DMTS and phenols, but higher esters and high alcohols, producing fruity clean spirit. Low reflux gives full, meaty, phenolic, robust new make. Esters apparently follow an U-curve, so that very low or high reflux produces more esters than an average one.
 |
| Lomond wash still in Scapa |
Just to put the theory to the test, below are the lyne arm angles and spirit still sizes of 24 Scottish malt distilleries compared against lightness-richness value given for the basic ~12yo malt of the distillery by Dave Broom in The World Atlas of Whisky. There is some, but not significant correlation between the lyne arm angle and the richness of whisky, but no correlation between the spirit still size and the perceived richness. Of course there are many other aspects affecting the products of the distilleries. Would have been too simple, if there was a clear correlation between either one...
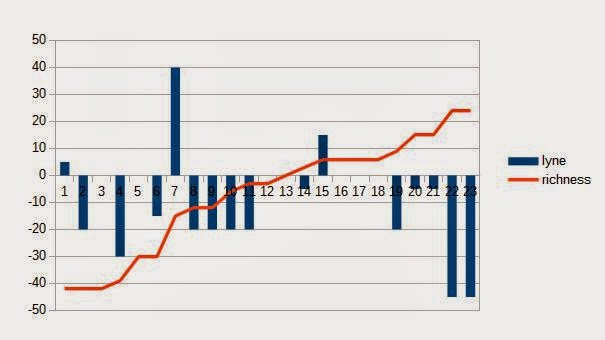 |
| Some correlation between lyne arm angle and richness of the basic OB |
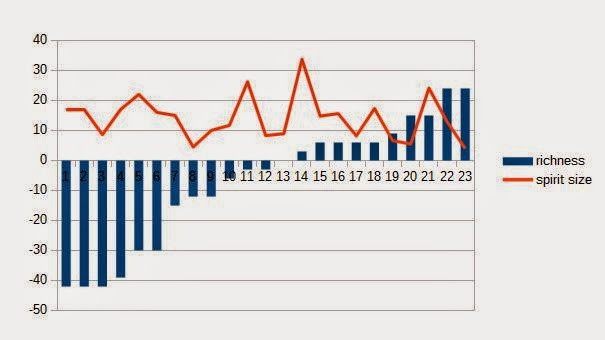 |
| No correlation between spirit still size and richness of the basic OB |
Alcarde A et al. Ethyl carbamate kinetics in double distillation of sugar cane spirit. J Inst Brew 2012;118;352-5
Broom D. World atlas of whisky. MItchell Beazley 2010
Bryce JH et al (ed). Distilled spirits: Production, technology and innovation. Nottingham Univ Press 2008
Forbes, RJ. Short history of the art of distillation. Brill 1948
Harrison, B et al. The Impact of Copper in Different Parts of Malt Whisky Pot Stills on New Make Spirit Composition and Aroma. J Inst Brew 2011;117(1);106-112
Hernández-Gómez L et al. Melon fruit distillates. Food Chem 2003;82;539-543
Jack, FR et al. Sensory implications of modifying distillation practice in Scotch malt whisky production. In Distilled Spirits, ed Bryce JH, Piggott JR, Stewart GG. Nottingham Univ Press 2008.
Lima U et al. Influence of fast and slow distillation on ethyl carbamate content and on coefficient of non-alcohol components in Brazilian sugarcane spirits. J Inst Brew 2012;118;305-8
Masuda, M and Nishimura, K. Changes in volatile sulfur compounds of whisky during aging. J Food Sci 1982; 47(1); 101-5
Monica Lee KY et al. Origins of flavour in whiskies and a revised flavour wheel. J Inst Brew 2001;107(5);287-313
Morewood S. A philosophical and statistical history of the inventions and customs of ancient and modern nations in the manufacture and use of inebriating liquors. Longman 1838.
Nedjma M, Hoffmann N. Hydrogen sulfide reactivity with thiols in the presence of copper in hydroalcoholic solutions or cognac brandies. J Agric Food Chem 1996;44;3935-38
Nóbrega I et al. Ethyl carbamate in cachaça. Food Chem 2011;127;1243-7
Prado-Ramírez R et al. The role of distillation on the quality of tequila. Int J Food Sci Tech 2005;40;701-8
Reaich, D. Influence of copper on malt whisky character. In Proceedings of 5th Aviemore Conference on malting, brewing & distilling. 1998
Riachi L et al. Review of ethyl carbamate and polycyclic aromatic hydrocarbon contamination risk in cachaça and other Brazilian sugarcane spirits. Food Chem 2014;149;159-169
Russell I. Whisky. Elsevier 2003.
Walker GM, Hughes PS (ed). Distilled spirits, new horizons: energy, environment and enlightenment. Nottingham Univ Press, 2010