 |
| Sulphur candle |
Sulphur in the whisky is mostly sourced from the aminoacids of the grains used in fermentation. The barley used in the whisky production are usually spring varieties and typically very low in protein and thus low on sulphury aminoacids (cysteine, methionine), too. However the yeasts metabolise the available aminoacids and in the process produce a variety of organic sulphur compounds. Typical byproduct of anaerobic sulphur metabolism is hydrogen sulphide (H2S), which has a strong unpleasant odor of rotten eggs and can further metabolise into thiols and other organic sulphur compounds. Excess amounts of yeast or the use of brewer's yeast or long fermentation times tend to increase the autolysis of yeasts and therefore add to the sulphur content of the wash. The lactic acid bacteriae can produce sulphury compounds, especially Lactobacillus brevis tends to impart a sulphury aroma.
Some sulphur is used during the kilning process, especially if peat is used to dry the grains. The anaerobic bacteriae in a peat bog produce sulphury compounds and obnoxious nitrosamines and by burning some sulphur with the peat the off-notes (and toxins) can be converted mainly to sulphur oxides, which do not spoil the grain.
The copper used in the distillation stills reduces the sulphury content of the whisky most likely by acting as a catalyst in processes resulting in insoluble copper sulphates. On the other hand copper has been associated with an increase in some sulphur compounds in the spirit, such as dimethylsulphate (DMS). Low copper contact (small/squat stills), fast distillation and high temperatures increase sulphury notes on new make spirit. Direct heating probably increases sulphury notes as there is bound to be some burning of grains at the bottom of the still and temperature variations between different parts of the still.
Cask maturation significantly reduces the amount of most sulphur compounds in the whisky, even so that in a recent study all of the dimethyl sulphide (DMS), 3-methylthiolpropylacetate, dihydro-2-methyl-3(2H)-thiophene and ethyl-3-methylthiol-propanoate had disappered after 3 years of oak maturation. Most organic sulphur compounds such as DMS decrease gradually during aging. This is most likely due to evaporation and to a lesser degree to oxidation or reactions with the carbon layer of the charred cask.
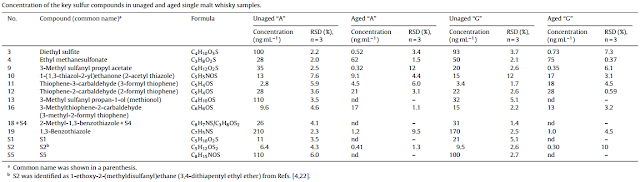 |
| Key sulphur compounds in new-make spirits and single malt whiskies (Masuda & Nishimura 1982) |
The individual perception of different sulphury compounds appears to be very different. As experienced tasters rated different sulphur compounds (in a study by Jack FR et al 2008), there was considerable variation between individuals and compound.
 |
| Sulphury character of different sulphur compounds, modified from Jack et al 2008 |
The most perceived MMFDS and 2-thiophene-cis-aldehyde, 4-methyl-thiazole, 4-methyl-5-vinyl-thiazole as sulphury and the mix of all was statistically the most sulphury of them all. At least one taster did rate the sulphury taste less than 1 out of 10 for all but two compounds. Several tasters were non-tasters for some compounds that the others rated highly sulphury. It is to be noted that all but one tasters rated one individual compound more sulphury than the mix of all, so the sulphury taste is not an add-on characteristic but rather a combination. For example 2-pentyl furan distictively suppresses the sulphury character of DMTS, just like salt suppresses a bitter taste (just try a tiny amount of salt in your coffee).
However, there has been controversy about sulphury casks in the whisky industry. Especially Jim Murray, the author of The Whisky Bible has been worried about sherry cask-derived sulphur-taints. Sulphur is widely used in wine industry to prevent bacterial growth in must and to improve the stability of wine. It is usually used in the form of sulphur dioxide, usually soluted in to a liquid form for ease of use. Sulphur dioxide acts as an antioxidant and antibacterial agent in wines. Excess sulphur dioxide content may intensify some allergic reaction and impart off-notes into wine. Sulphur candles or brimstone sticks have been used to preserve casked wine and to prevent bacterial contamination of casks stored empty.
Fumigation of casks with sulphur has been used probably from the Roman era. The use of sulphur matches and candles for preserving wines and other perishables was common in late 18th century Europe. Wine writer André Jullien describes the fumigation of wine casks in 1825:
"Fumigating wines is impregnating them with sulphurous vapours, obtained by the burning of brimstone matches... aromatics are often mixed with the brimstone... the Strasbourg [violet scented matches] are to be preferred for wine... When old wine runs clear, it is sufficient to burn a bit of match in the cask you are going to fill. To hasten the fermentation of new wine, burn several matches and shake the wine in the vapour... Many vineyards produce wines of a sulphurous taste, which goes off in time" (as cask maturation/storing was common at the time). This practice reduced the oxygen in the cask and prevented lactic bacterial brettanomyces contamination, therefore enhancing the stability and quality of wine.
 |
| Sulphur burners are still used by amateur winemakers. |
The effects of sulphur in casks were not completely understood and in 1873 there was a scandal in Britain, as Dr Thudicum wrote that the sulphuring, plastering (adding calciumcarbonite into must) and fortification of sherry was to be considered as adulteration and that the sherry wines were inferior to the French wines and probably dangerous to health. The fact that also the French were sulphuring their casks was not discussed and there were probably some trade-oriented motives behind the argument.
 |
| Different types of sulphur used in winemaking |
Since 1986 Spain has been a member of EEC and the shipment of sherry has been made very hard by the Denominacion de Origen to encourage bottling in Spain. Bottling of sherry is done almost exclusively in Spain and full sherry casks are no longer imported. The sherry shippers had already started their own bottling plants in Spain in the early 20th century. Pedro Domecq started their bottling operations in Jerez in 1920 and Gonzalez Byass was to follow gradually during the interwar period. Sandeman bottled some of their sherries and ports in location as early as 1880, but the bottling of sherry in England by Sandeman ceased in 1969. Harvey's were the last big shipper to bottle their sherries in England, as they bought a winery in Jerez from MacKenzie in 1970 and since then have been bottling practically all of their sherries in Spain. Therefore since early 1970s many distillers have been maturing whisky in sherry casks made to order in Spain. Both American and Spanish oak casks are coopered and usually the sherry used in seasoning is oloroso, but sometimes lower quality blending sherry called raya, which resembles oloroso. Some bodegas, for example Gonzalez Byass and Pedro Romero trade their old empty solera casks, which are made exclusively of American oak and usually 80-100 years old and probably very different from a typical sherry shipping cask or a seasoned cask. Another quite popular way of producing sherry casks was to rejuvenate old exhausted cask by scraping the inner surface, toasting it again and seasoning it with sherry. Aeriation of whisky, during bottle maturation or in greater extent after the bottle has been opened, usually decreases the highly volatile sulphury notes.
In conclusion, there is good and bad sulphur in whisky. To simply pin one or two sulphur compounds responsible of the good or the bad aromas would be an oversimplification. Similarily the origins of sulphury notes seem to be impossible to track to just one source, such as sulphury cask and there is no evidence of excess use of sulphur candles in the sherry industry during the last decades, in fact quite the opposite. More likely is that there are some bad batches distilled too fast or in too warm climate that are over-sulphury, or maybe a cask has not been properly sulphured and has been contaminated with brettanomyces.
REFERENCES AND FURTHER READING
Harrison, B et al. Impact of copper in different parts of malt whisky pot stills on new make spirit composition and aroma. J Inst Brew, 2001;117(1);106-112
Jack, FR et al. Sensory implications of modifying distillation practice in Scotch malt whisky production. In Distilled Spirits, ed Bryce JH, Piggott JR, Stewart GG. Nottingham Univ Press 2008.
Jack, FR. Understanding Scotch whisky flavour. Food Sci Tech 2003;14;28-30
Jullien, A. Wine merchant's companion and butler's manual. 1825
Labuza, T et al. Maillard reactions in chemistry, food and health. RSC 1994.
Masuda, M and Nishimura, K. Changes in volatile sulfur compounds of whisky during aging. J Food Sci 1982; 47(1); 101-5
Reaich, D. Influence of copper on malt whisky character. In Proceedings of 5th Aviemore Conference on malting, brewing & distilling. 1998
www.practicalwinery.com/janfeb09/page1.htm
REFERENCES AND FURTHER READING
Harrison, B et al. Impact of copper in different parts of malt whisky pot stills on new make spirit composition and aroma. J Inst Brew, 2001;117(1);106-112
Jack, FR et al. Sensory implications of modifying distillation practice in Scotch malt whisky production. In Distilled Spirits, ed Bryce JH, Piggott JR, Stewart GG. Nottingham Univ Press 2008.
Jack, FR. Understanding Scotch whisky flavour. Food Sci Tech 2003;14;28-30
Jullien, A. Wine merchant's companion and butler's manual. 1825
Labuza, T et al. Maillard reactions in chemistry, food and health. RSC 1994.
Masuda, M and Nishimura, K. Changes in volatile sulfur compounds of whisky during aging. J Food Sci 1982; 47(1); 101-5
Reaich, D. Influence of copper on malt whisky character. In Proceedings of 5th Aviemore Conference on malting, brewing & distilling. 1998
www.practicalwinery.com/janfeb09/page1.htm

Txh for feeding us more informativ and helpful stuff!
ReplyDeleteBjörn Scholz
Thanks for this very informative post. But there was a little mistake where it says "The copper used in the distillation stills reduces the sulphury content of the whisky most likely by acting as a catalyst in processes resulting in insoluble copper sulphates."
ReplyDeleteAs catalyst copper speeds up any of those reactions, but doesn't get changed itself. That means, none of these reactions can result in copper ions which are necessary for copper sulphates. Secondly, copper sulfates are highly soluble in water what results in a very deep blue and or turquoise color of the liquid.
Nevertheless thanks for the work!
I think he is saying CuO. Cu(Copper) doesn't react with alcohol, but it and be oxidized by oxygen and form CuO. CuO react with alcohol by heat and resulting in acetaldehyde. And by the way, Copper do react with sulfur.
ReplyDelete2Cu + O2 =HEAT= 2CuO
CH3CH2OH + CuO =HEAT= CH3CHO + Cu + H2O
[Cu(NH3)4]2+ + H2S + 2H2O = CuS↓ + 2NH3·H2O + 2NH4+
H2S
I think we need the chemical expertise of Henric Molin from Spirit of Hven Backafallsbyn - he is a master distiller and master chemist!
ReplyDelete